On Aug. 18, 2053, Tyson Foods unveiled its much-anticipated product, Well Beef, at a benefit dinner in Lower Manhattan. Well Beef, a genetically engineered animal product derived from what the company is calling “Welfare-Enhanced Cows,” is the third GE food product that Tyson has released and comes just a year on the heels of Ecopig.
“It’s exceptional!” exclaimed Grant Willis, the company’s CEO, dabbing at his chin with a napkin. “We have finally achieved the Big Three. We have enjoyed phenomenal success with Pure Chicken and Ecopig, and now we are ushering in the era of Well Beef.” Willis gestured toward the room, where guests eddied about the tables surveying and sampling Tyson’s array of genetically engineered foods. The chefs had transformed the Well Beef into carpaccio woven into the shape of roses. Interspersed were silver trays of Ecopig sliders and Pure Chicken pâté nestled among garlands of fruit and salad greens.
As Pure Chicken celebrates half a decade of success this year, Well Beef is the first commercially available GE beef product that claims to be cruelty-free. “Genetic engineering for increased welfare” boasts a banner draped behind the podium. Light from the chandeliers shimmered across the marbled beef, giving the meat a dewy sheen. Lush bouquets of flowering sweet pea vines were placed throughout the rooms. “An homage to trait selection,” Herbert Muller, an attending geneticist from Tyson, tells me. “On this day, it is worth reflecting on humanity’s journey through the labyrinth of heredity.”
Unlike these pea vines, whose differences are largely cosmetic, what makes Well Beef distinct from traditional meat goes beyond appearances. By using CRISPR and other gene editing technology, companies like Tyson can make targeted modifications to the genome in order to delete or insert new genes. While those who selected favorable variants in the past were doing so in ways that were molecularly indiscriminate, today’s food engineers have gone from merely interpreting the gene to manipulating it.
This kind of genomic selection was first introduced into the dairy industry in 2009, where it was adopted rapidly. By 2015, more than half of all artificial insemination matings in the United States were made to genomically tested young bulls, resulting in cows that were larger and produced more milk. But even in this era, the gene was not being changed at its core, only selected for. It took decades of advancements in molecular technologies before this kind of targeted genomic selection could be made more efficient.
“When I was working in agricultural biotech back in the mid-2020s, things were different,” says Vivian Carver, another attendee, a retired researcher at the Institute for AgriGenetic Welfare. “We had the technology to edit the genome but regulatory gridlock had us in a chokehold. Every meal we have ever consumed is genetically distinct from every other meal and yet explicitly editing a gene made regulators pounce.” For example, under the FDA’s 2009 Guidance for Industry entitled “Regulation of Genetically Engineered Animals Containing Heritable Recombinant DNA Constructs,” any organism that was modified with rDNA techniques was viewed as containing “drugs” and regulated as such; a single deletion in the genome triggered strict regulatory inspection.
This meant that GE research was subjected to strict oversight — even when the part of the animal being modified had no direct connection to the part of the animal consumed or producing food. For example, researchers attempting to shorten cattle horns to eliminate the need for painful dehorning practices were forced to present evidence that alterations in the horns would not affect the milk. Unsurprisingly, the milk was indistinguishable. Many scientists pushed back. After getting rejected for a grant back in 2019, former biotech researcher Alison van Eenennaam and her colleagues published their protestations in Nature Magazine, asking, “How can the absence of a small piece of DNA rationally be considered a drug?”
Other scientists, including Carver, simply left. Dismayed by the opportunity costs of adhering to regulatory restrictions, many joined or established research centers throughout Central and South America, where regulations on animal research were minimal or non-existent.
Thus, the first two decades of the 21st century were somewhat of a dark age for GE livestock, particularly in the United States. Only a few products like AquAdvantage Atlantic Salmon (which had already sat in regulatory limbo for over twenty years) were able to fight their way to commercial success. Many projects stalled and agriculture futures bottomed out. While GE animals floundered, yeast- and plant-based protein enjoyed their zenith. The popularity of plant-based diets exploded across wealthy nations, though the rate of overall meat consumption hardly dipped due to steadily growing wealth and populations in developing nations. Cultured meat also failed to take off in the way that advocates for alternative protein had hoped. Despite scientific advancements, scaling up cultured meat enterprises proved remarkably challenging and expensive.
However, in retrospect, the livestock revolution was beginning to happen quietly. Improvements geared toward the welfare of livestock and animal products began at the margins. Early advancements weren’t conducted under the banner of animal welfare, but the most pressing public fears of the time: climate change and pandemics. Initial gene modifying efforts were directed at disease resistance or climatic adaptations. Early in the 2020s, agricultural researchers selectively bred cattle to have shorter hair to help them regulate heat and engineered PRRS-virus-resistant pigs. Emboldened by these successes, scientists continued to push the capabilities of genetic engineering even as they faced funding constraints and regulatory overreach.
It was in the 2030s that the tide truly began to change. Efforts to abolish intensive animal agriculture continued to fail. States that tried to shift their agricultural production away from livestock faced exacting retribution. After the court ruled in favor of concentrated animal feeding operations (CAFOs) in the landmark 2037 court decision Cal-Maine Foods, Inc. v. Iowa, it appeared that the meat lobby was too strong. However, their influence was soon to be curtailed. After a wave of zoonotic pandemics such as H1N1-39 broke out in the 2040s, the National Pork Producers Council and their subsidiaries embarked on a frenzied crusade to rehabilitate their public image, ultimately shaping the trajectory of today’s livestock industry.
Facing unrelenting political pressure stoked by the public’s concern over zoonotic pathogens, CAFO lobbies were forced to compromise. In a series of unprecedented negotiations, companies like Cal-Maine and Tyson sat down with advocates from the Humane League and the National Institute of Allergy and Infectious Disease to discuss the future of meat production. If consumers and corporations refused to give up meat entirely, there had to be another solution for those demanding animal welfare and human safety.
The upshot was monumental: CAFOs could continue to operate as long as they worked toward disease and pathogen resistance — a determination that called upon genetic engineering as an engine of compromise.
“Although for many it was sad to see technology succeed where moral arguments had not, it seemed the second-best solution,” conceded bioethicist and historian Caldwell Marin during an interview. “The meat industry was placated, and those who cared about the welfare of humans and animals were emboldened to make real change.”
For decades, the rift between those working within animal agriculture and those fighting for an overhaul of factory farming led both sides to weaponize gene-editing technology against each other. The narrative was that GE could either be aimed at efficiency or at welfare, but never at both. However, the incompatibility of efficiency and ethics proved false, and once these interests were aligned, the necessary technology emerged rapidly.
The 2040s brought about the GE renaissance. Fearful of the opportunity costs of delaying transgenically modified organisms after the post-pandemic negotiations of the ’40s, CAFOs increased their pressure on regulatory bodies like the FDA. This pressure, alongside the bold promises for pathogenetically resistant livestock by the National Institute of Allergy and Infectious Disease, finally prompted the FDA to initiate a regulatory overhaul. With it came a deluge of talent and resources, and it wasn’t long after that researchers like Carver flocked back to the United States. Still held to rigorous standards with respect to toxicity and allergenicity, the language and simplified reporting specifications in the new Guidance for Industry allowed for easier R&D. Finally unimpeded, biotech researchers could push the boundaries of genetic engineering — from changing an animal’s experience of heat to altering its ability to experience entirely.
Tyson’s Pure Chicken was the first GE animal engineered not to perceive pain. Using CRISPR and other proprietary technologies, bioengineers were able to manipulate the chickens so they had brain function sufficient for maintaining growth but not for supporting mental states or psychological experiences. These chickens, which lacked beaks, eyes and feathers, also had ablations to their anterior cingulate that disrupted the affective dimensions of pain. Their secondary somatosensory cortex was left intact, rendering them able to eat and drink, and even to react instinctually to stimuli. But when exposed to adverse stimuli, rather than exhibiting nociceptive behavior, they remained serene. They resembled something between an animal and a fruit, an observation that is encapsulated by the product’s official slogan: “They may as well grow on trees.”
Although the product had its critics, its immediate commercial success left no doubt about the industry’s trajectory. Within months of its debut in 2048, Pure Chicken became the industry standard. Non-genetically engineered chicken simply could not compete with Pure Chicken for taste or efficiency. And whereas traditionally bred chickens are prone to pecking one another’s eyes out when too tightly confined, Pure Chickens are equanimous and placid. From temperament to taste, cruelty-free chicken outmatched non-GE poultry.
In the ebullience that followed the commercial success of Pure Chicken, companies like Tyson and Cal-Maine Foods turned their attention to bioengineering a larger array of more complex livestock animals. The methods that researchers used were similar, focusing on disrupting the neural pathways so that they could alleviate pain while not stunting growth. Three years ago, in the spring of 2050, a team of animal science researchers from California Polytechnic State University discovered that folic acid deficiency during embryogenesis could lead to a neural tube defect that disrupts pain signaling in the brain. They first implemented this strategy in pigs, which led to a spate of GE pork products, including Ecopig, before turning their attention to modifying cattle.
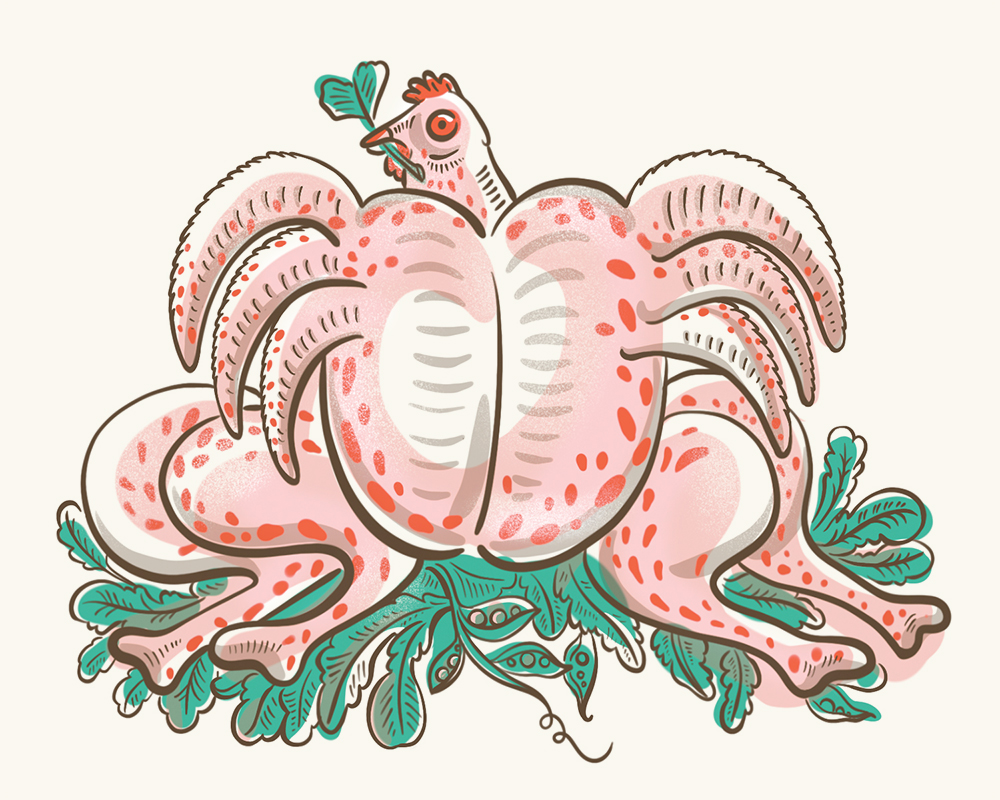
Well Beef is thus the tour de force of GE livestock. The welfare-enhanced cows from which Well Beef is manufactured are a genetic hybrid of Holstein and Angus cattle. Large and muscle-bound, their architectonic bodies ripple with prime cuts. The most pronounced distinction between these beef cows and their forebears is their heads, which develop with a concave brain but retain a partial skull, including the face.
According to Tyson, these cattle eat, grow, live and die without a vestige of pain. Even the most skeptical evaluators confirmed this appraisal. Upon visiting Tyson’s headquarters last month, Maxwell Harder, an investigator with the Factory Farming Awareness Coalition, marveled that he had “plausibly borne witness to the largest reduction of suffering ever undertaken.”
But some neuroscientists and bioethicists remain skeptical.
“The brain is an astonishingly complex organ,” said neuroscientist Masha Ruhig of the Center for Neuroscience and Society. “Millions of neurons throughout our brain signal pain. These neurons are spread about in every region. Many of my colleagues are concerned that once we ablate the regions of the brain that are connected to the conscious recognition of pain that the brain will simply reforge these connections elsewhere.”
In most cases, scientists celebrate the brain’s ability to compensate for damage. But in the case of GE livestock, this kind of neuroplasticity could put an end to the industry’s optimism. The billions of farm animals undergoing genetic modifications so that they cannot experience suffering might still feel pain in a manner that evades our current understanding of neuroscience. Researchers and ethicists like Ruhig fear that with the absence of a fully formed brain, the neural signals of these GE animals will simply reemerge in different regions. In doing so, neural pain responses might evade our detection.
In other words, genetically engineered animals might still experience pain because bioengineers are either wrong about how to disrupt the pain response or because the conscious recognition of pain takes place somewhere unanticipated.
“I certainly don’t want to be a prophet of doom,” continued Ruhig. “If the bioengineers at places like Tyson are correct and these pain receptors really don’t migrate, then what they have achieved is epoch shaping.” If they’re wrong, the industry will find itself not far from where it started in terms of the moral implications.
I recall her solemnity as the benefit dinner concludes with rousing addresses from Willis and others from Tyson outlining the turbulent history of genetically engineered livestock and speaking with rapture about developments to come. Alan Park, the company’s program director, passionately details a pilot program geared toward growing animal limbs from a central node containing a slurry of nutrients and DNA. “Think of it as a vertical bestiary!” he says, smiling.
“Animal agriculture need not be stuck in the past,” Park continues. “And thanks to our scientists’ groundbreaking achievements, the contradiction between eating meat and eating ethically has vanished.” His address evokes deafening applause.
A gentle breeze blows through the dining room, rustling the chandeliers and sending napkins up into the air. Park steps down from the lectern and osmoses into the audience between a flurry of handshakes. A waiter emerges beside me and offers me a tray of Well Beef. I take a sample, dropping the parsley garnish into my napkin.
The meat melts against the roof of my mouth, marbled, succulent. It might be my imagination, but I can taste the sun-baked pastures of middle America, lands no longer grazed upon by sufferers, but by the beneficiaries of scientific advancement.